We use techniques in molecular biology, behavioural neuroscience, and
functional genomics to study phenotype. A major focus of our lab is the
role of epigenetic mechanisms that confer inter-individual variation in
glucocorticoid signaling pathways. Social factors, infections, toxins
and disease can all influence gluccorticoid signaling. As such,
glucocorticoid signaling pathways act as sensors for environmental
signals that affect a range of physiological functions from the response
to stress to inflammation. Another major focus in the lab is the role of
early life environmental adversity in altering brain and behaviour later
in life via epigenetic mechanisms. We study a variety of organisms, from
animals in the lab to wild populations and humans.
Our research is funded by grants from the Natural Sciences and
Engineering Research Council of Canada (NSERC), the Canadian Institutes
of Health Research (CIHR), the Connaught Fund, the Solve ME/CFS
Initiative, the Falk Medical Research Trust and the United States
Department of Defense.
Our Research
Credit: Daniel Horowitz for NPR
Epigenetics
The term epigenetic literally means "above the genome". The main
components of the epigenetic code are DNA methylation, histone
modifications, and non-coding RNAs. The epigenetic programs in cells
are normally faithfully reproduced during mitosis and maintained in
the post-mitotic cell. They can also be maintained during meiosis,
resulting in epigenetic transgenerational disease inheritance, and
potentially introducing phenotypic variation that is selected for in
the evolution of species.In the broadest sense of the term, epigenetic changes are all changes in gene function that do not involve a change in the underlying sequence of the gene. Epigenetic changes can switch genes on or turn them off, or modify the way in which genes are transcribed. Whereas some epigenetic modifications are heritable (passed through the germline), others can persist throughout the life of the organism.
DNA Methylation
DNA methylation is a covalent bond between a methyl group and, in
mammals, the 5’ cytosine ring of the dinucleotide combination CG.
Because it involves a covalent bond, DNA methylation is a very stable
epigenetic modification. DNA methylation in the promoter regions of
genes is usually associated with the silencing of gene expression.
Imprinted genes are examples of epigenetic control by DNA methylation,
where one allele - either from the mother or from the father - is
switched off by DNA methylation.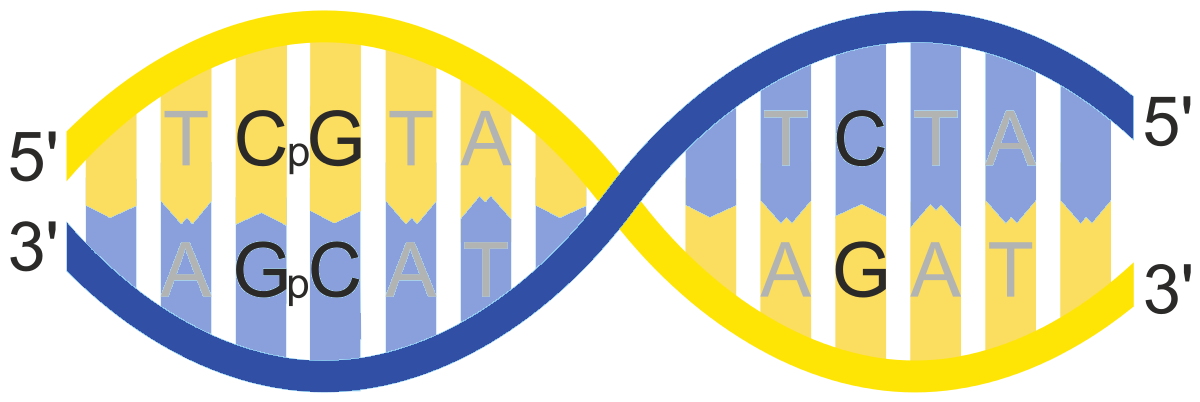
Epigenetics in Health & Disease
The health of organisms from plants to animals to humans depends upon
proper epigenetic control. Malfunctions in the epigenetic machinery
during development have been linked to several major pathologies in
humans, including chromosomal instabilities (e.g., Fragile-X), and
mental retardation (e.g., RETT syndrome). There are now compelling human epidemiological and animal experimental data that indicate the risk of developing complex diseases is influenced by persistent epigenetic adaptations in response to early exposures to environmental factors, including toxins, nutrition, and the social environment. Environmental exposures during development may alter the risk of developing medical conditions such as asthma, autism, cancer, cardiovascular disease, diabetes, and obesity by modifying the epigenome. Epigenetic changes during development have also been linked to psychiatric disorders, including schizophrenia, bipolar disorder, and depression. Because epigenetic changes involve modifications to gene function rather than changes to gene sequence, they are potentially reversible, and amenable to therapeutic intervention.